Abstract
Background: Venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication of both cancer and its treatment.Unlike low-molecular weight heparins (LMWHs) which represent the current standard-of-care for cancer-associated venous thromboembolism (CAT) management, rivaroxaban can be administered orally, avoiding the need for uncomfortable injections. While not guideline-recommended as first-line therapy for CAT, rivaroxaban has been used in routine clinical practice for this purpose.
Objective: To estimate the cumulative incidence of recurrent VTE, major bleeding and mortality in patients with CAT treated with rivaroxaban as outpatients in routine clinical practice.
Methods: Within US Truven MarketScan administrative claims data from 1/2012 to 6/2015, we identified adult patients with active cancer using National Cancer Institute-Surveillance, Epidemiology and End Results Program (NCI-SEER) coding who had ≥1 primary hospitalization or emergency department visit discharge diagnosis code for DVT and/or PE (the index event) and received a prescription of rivaroxaban as their first outpatient anticoagulant within 30-days of the index VTE. Eligible patients were required to have a minimum of 180-days continuous medical and prescription benefits prior to the index VTE (baseline period). Patients with a prior claim for VTE or receiving anticoagulation during the baseline period were excluded. We estimated the cumulative incidence with 95% confidence intervals (CIs) of recurrent VTE, major bleeding (identified per the Cunningham algorithm) and mortality (defined as in-hospital mortality or need for hospice care without subsequent claims) at 180-days assuming competing risks.
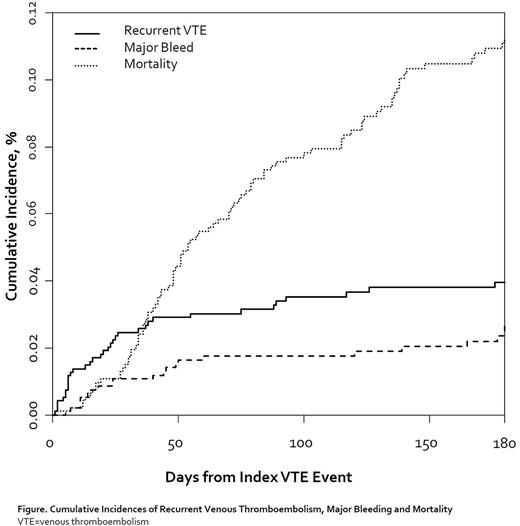
Results: A total of 949 active cancer patients were initiated on rivaroxaban following their index VTE. The mean ± standard deviation (SD) time from index VTE to first outpatient rivaroxaban fill was 4.4±5.8 days. Mean±SD age of identified patients was 62.5±12.8 years, 45.6% were men and PE (with or without DVT) was present in 43.6% of patients. Time from the diagnosis of active cancer to index CAT was ≤90-days in 30.0%, 91-180 days for 18.9% and 181-days of greater for 54.3% of patients. Cancer sites for this rivaroxaban-treated cohort were as follows: 25.5% breast, 21.0% genitourinary (including prostate), 13.2% hematologic, 13.2% colorectal, 11.5% lung, 7.4% gynecologic, 5.8% pancreas, 4.1% stomach/esophagus and 39.5% other. Metastatic disease was present in 42.6% of patients. During follow-up, there were 37 cases of recurrent VTE, 22 cases of major bleeding and 105 deaths. The 180-day cumulative incidence estimate was 4.0% (95%CI 2.8-5.4%) for recurrent VTE; 2.7% (95%CI=1.7−4.0%) for major bleeding and 11.3% (95%CI=9.2-13.6%) for mortality (Figure).
Conclusion: Event rates observed in this rivaroxaban treated cohort were consistent with those observed in prior studies of LMWH- and rivaroxaban-managed CAT patients. Our data may provide some reassurance to clinicians regarding the effectiveness and safety of rivaroxaban in treating CAT patients in routine outpatient practice.
Beyer-Westendorf: Pfizer: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria, Research Funding. Spyropoulos: Boehringer Ingelheim: Consultancy; Janssen Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Portola: Membership on an entity's Board of Directors or advisory committees. Coleman: Boehringer Ingelheim: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bayer AG: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal